There is a tremendous need for new strategies to promote the regeneration of musculoskeletal, skin, vascular and dental/oral/craniofacial tissues, due to the large number of patients suffering from disease or trauma to these tissues. We are taking a variety of approaches to meet this need, with many revolving around the use of biomaterials that either serve as carriers for morphogens or cells that can participate in regeneration, and others exploiting mechanical cues (see Mechanotransduction and Mechanotherapy section) (Fig. 1). These materials can typically be introduced into the body using minimally invasive techniques (e.g., injection), and regulate in time and space the presentation of molecular cues and subsequent recruitment of host cells that orchestrate regeneration.
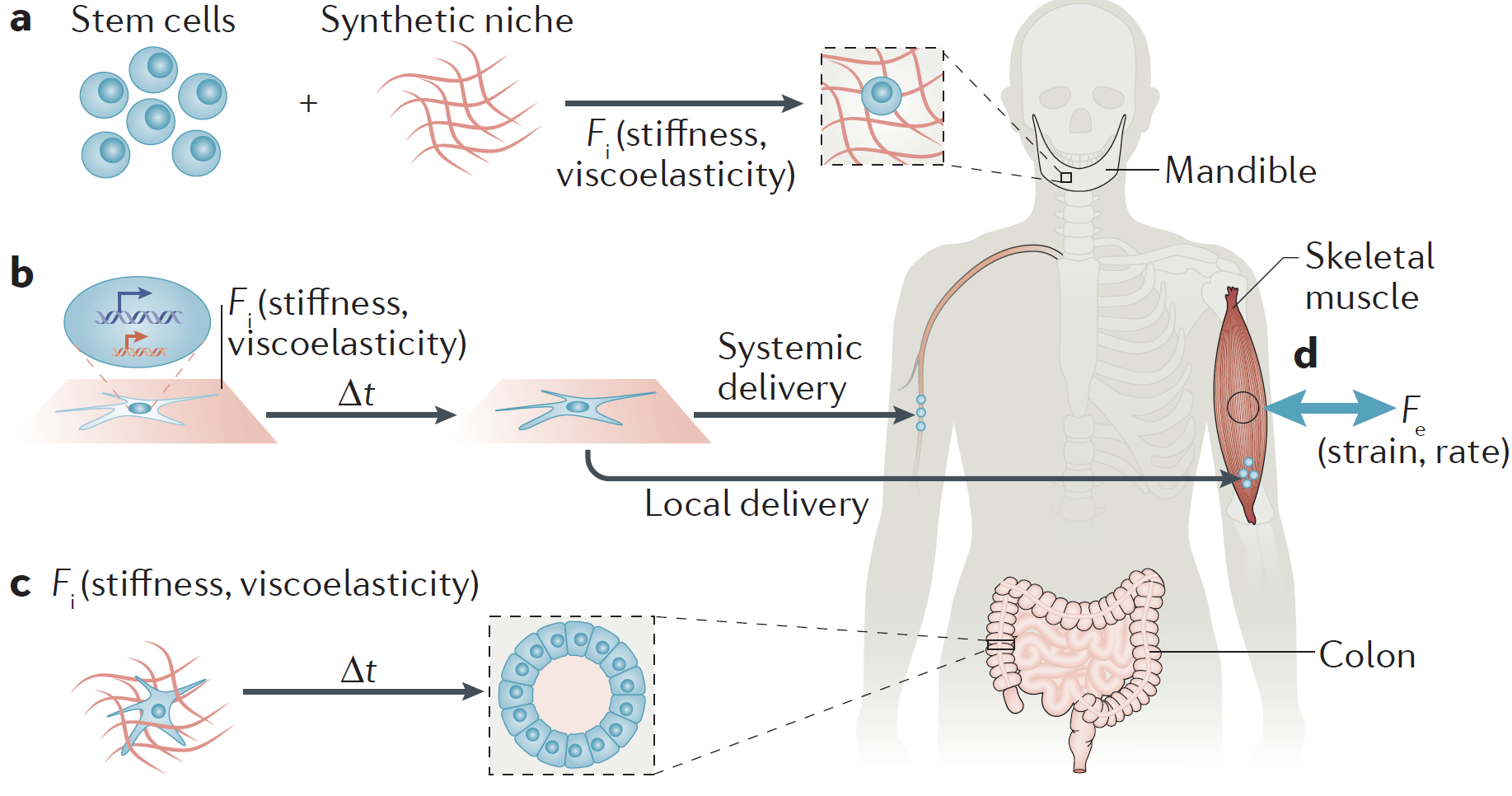
Figure 1. Tissue regeneration can be improved by using biomaterials to exploit cell biology. Image taken from (Vining, KH et al., Nature Molecular Cell Biology, 2017).
Biomaterial-assisted cell delivery:
To address the current limitations of cell transplantation in rebuilding tissues (e.g., large-scale cell death), we have been designing biomaterial carriers that maintain cell viability during transplantation while subsequently stabilizing the cells or mobilizing to disperse and engraft through a large tissue volume in vivo (Fig. 2). Additionally, we are exploiting our expertise in drug delivery to create new networks of blood vessels within regenerating tissues, and reestablish innervation as capillary networks are built.
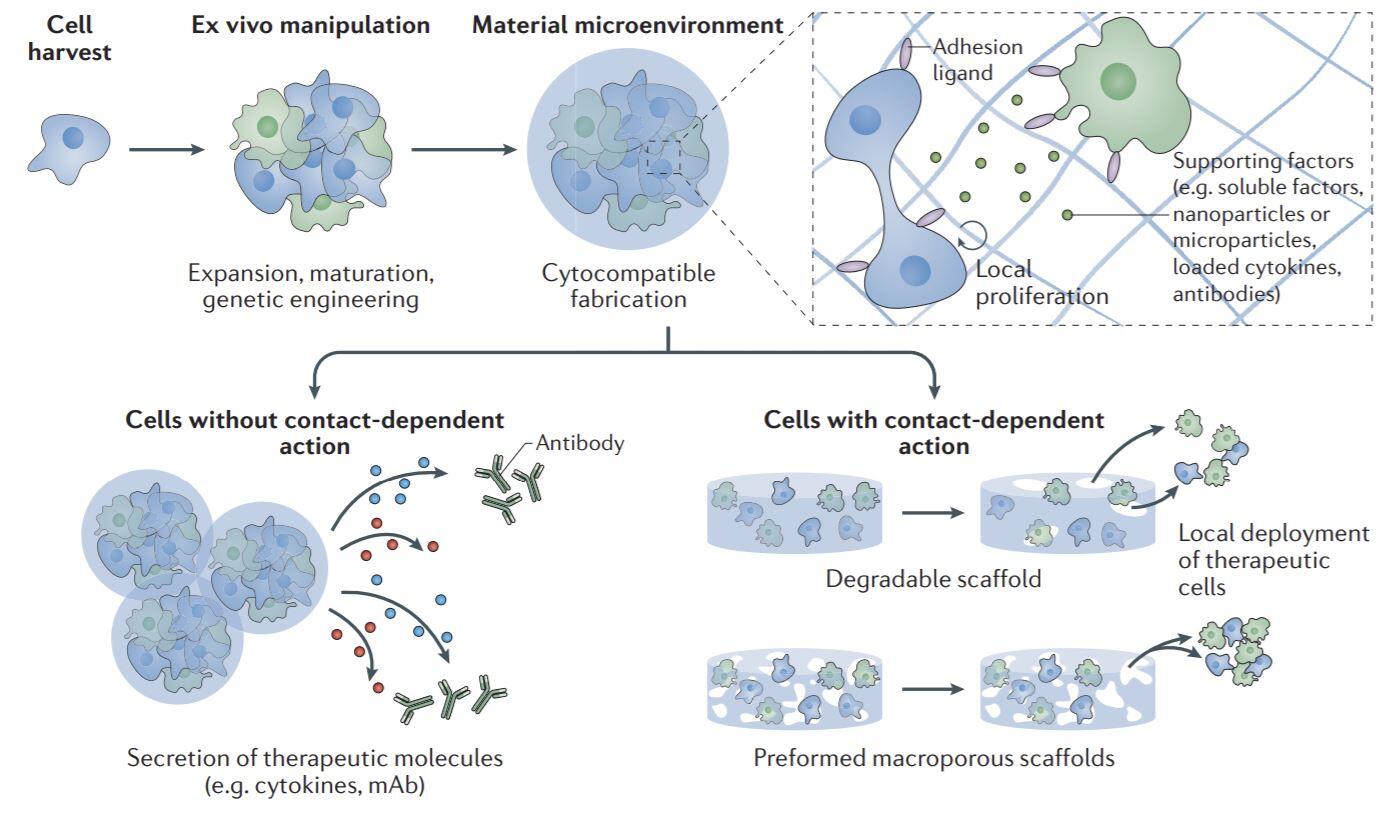
Figure 2. Biomaterial-assisted cell delivery. Cells harvested for therapeutic delivery can be expanded ex vivo and modified as needed before being precisely delivered using biomaterials. Image taken from (Dellacherie, Seo et al., Nature Reviews Materials, 2019).
Biomaterial niches for host-cell modulation:
It is clear that cells associated with both innate and adaptive immunity play key roles following tissue damage, and control over their numbers and functional states may allow one to promote regeneration (see Immunotherapy and Immunoengineering section). We are developing biomaterial systems to recruit host cells and modulate them to promote regeneration (Fig. 3). For example, we are investigating how biomaterials can be leveraged to regulate the frequency and polarization state of macrophages at a site of tissue damage, and are exploring how this impacts the regeneration of skin and muscle. The role of various T cell populations in angiogenesis and regeneration are similarly being explored using biomaterials that regulate the number and type of T cells, and the impact of antigen-specific immune responses in these processes are being explored.
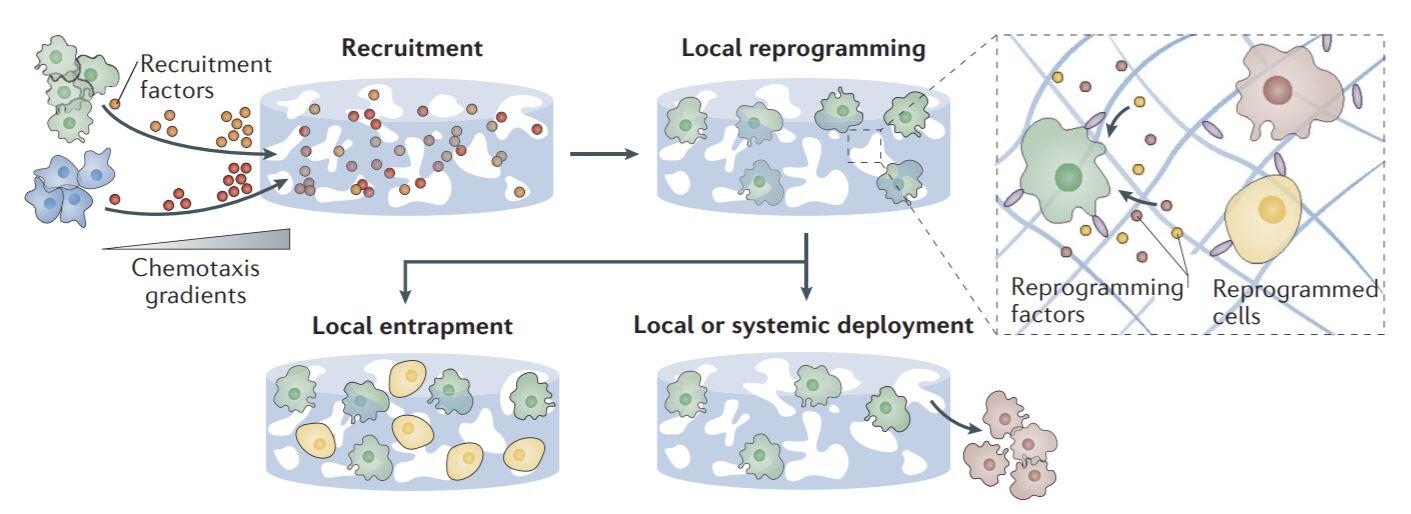
Figure 3. Biomaterial niches for host–cell modulation. Biomaterial niches are used to modulate cellular behaviour without ex vivo manipulation. Image taken from (Dellacherie, Seo et al., Nature Reviews Materials, 2019).
Relevant review articles in this area
- Dellacherie, M.O., Seo, B.R. & Mooney, D.J. Macroscale biomaterials strategies for local immunomodulation. Nat Rev Mater 4, 379–397 (2019). https://doi-org.ezp-prod1.hul.harvard.edu/10.1038/s41578-019-0106-3
- Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci. 2015;112(47):14452-14459. doi:10.1073/pnas.1508520112.
- Kwee BJ, Mooney DJ. Manipulating the intersection of angiogenesis and inflammation. Ann Biomed Eng. 2015;43(3):628-640. doi:10.1007/s10439-014-1145-y.
Representative research publications in this area
- Shah, N.J., Mao, A.S., Shih, TY. et al. An injectable bone marrow–like scaffold enhances T cell immunity after hematopoietic stem cell transplantation. Nat Biotechnol 37, 293–302 (2019). https://doi-org.ezp-prod1.hul.harvard.edu/10.1038/s41587-019-0017-2
- Raimondo TM, Li H, Kwee BJ, Kinsley S, Budina E, Anderson EM, Doherty EJ, Talbot SG, Mooney DJ. Combined delivery of VEGF and IGF-1 promotes functional innervation in mice and improves muscle transplantation in rabbits. Biomaterials. 2019 Sep;216:119246. doi: 10.1016/j.biomaterials.2019.119246.
-
Kwee BJ, Seo BR, Najibi AJ, Li AW, Shih TY, White D, Mooney DJ. Treating ischemia via recruitment of antigen-specific T cells. Sci Adv. 2019 Jul 31;5(7):eaav6313. doi: 10.1126/sciadv.aav6313.
-
Mao AS, Özkale B, Shah NJ, Vining KH, Descombes T, Zhang L, Tringides CM, Wong SW, Shin JW, Scadden DT, Weitz DA, Mooney DJ. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc Natl Acad Sci U S A. 2019 Jul 30;116(31):15392-15397. doi: 10.1073/pnas.1819415116.
- Mao AS, Shin J-W, Utech S, et al. Deterministic encapsulation of single cells in thin tunable microgels for niche modelling and therapeutic delivery. Nat Mater. 2017;16(2):236-243. doi:10.1038/nmat4781.
- Anderson EM, Silva EA, Hao Y, et al. VEGF and IGF Delivered from Alginate Hydrogels Promote Stable Perfusion Recovery in Ischemic Hind Limbs of Aged Mice and Young Rabbits. J Vasc Res. 2017;54(5):288-298. doi:10.1159/000479869.
- Borselli C, Storrie H, Benesch-Lee F, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci. 2010;107(8):3287-3292. doi:10.1073/pnas.0903875106.
- Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci. 2002;99(19):12025-12030. doi:10.1073/pnas.192291499.

