We focus on a multiscale design of drug delivery systems, to provide versatile platforms to meet specific application-based requirements.
We are interested in the development of different polymer systems (i.e., hydrogels) that allow for minimally invasive delivery (injectable systems) or implantable systems for various applications in musculoskeletal, skin and oral/craniofacial regeneration, and cancer therapy (Fig. 1). These systems, which deliver therapeutic agents (e.g., cells, protein growth factors, chemoattractants, antibodies, genetic material, etc.) are being tested in a variety of small/large animal models, and human clinical trials for various applications.
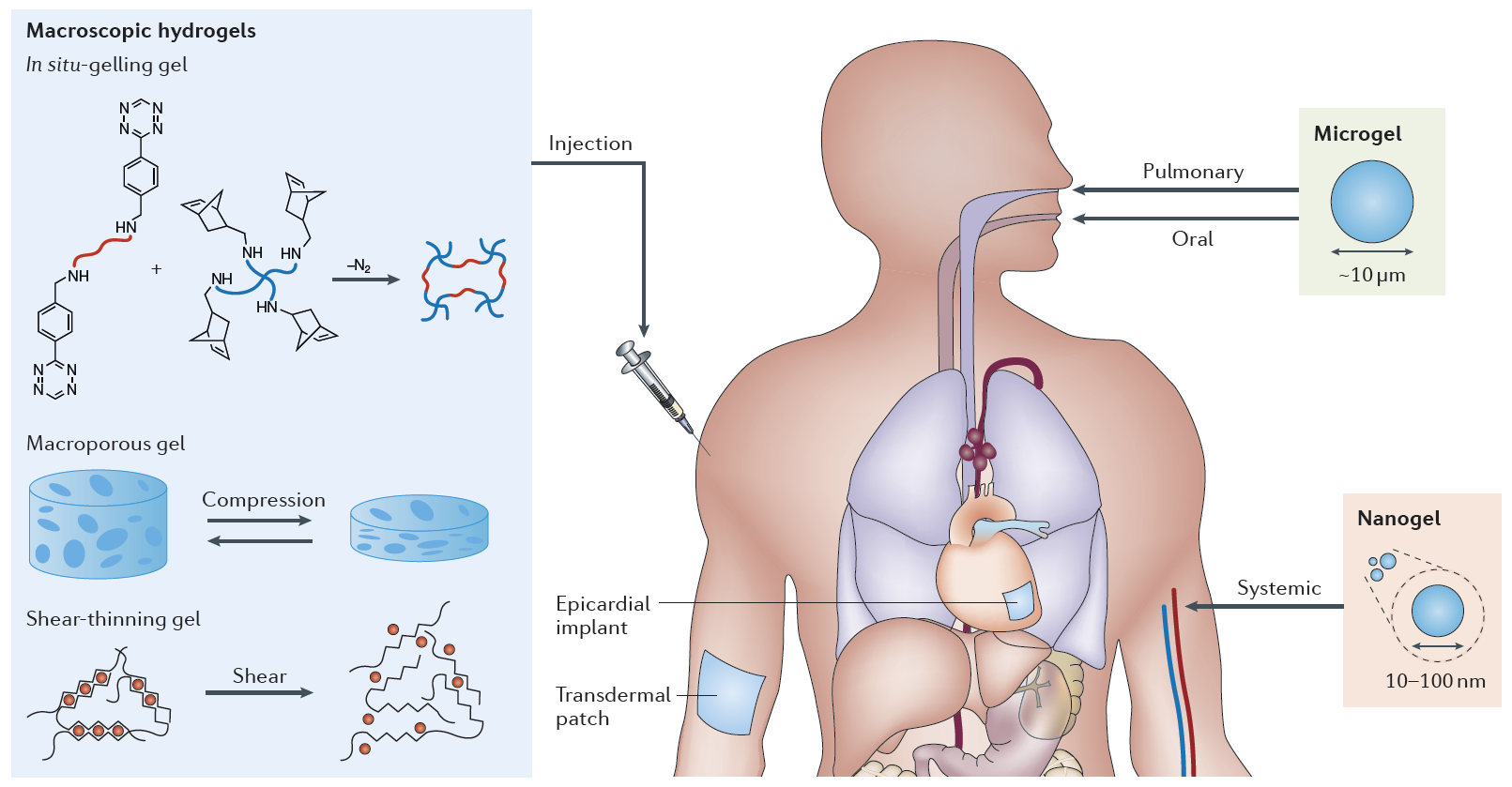
Figure 1. Drug-laden polymeric hydrogels of varying size can be delivered to the body in a minimally-invasive manner for therapeutic applications. Image taken from (Li, J. et al., Nature Reviews Materials, 2016).
Reloading of delivery devices in situ, and dynamic, real-time control over delivery are of major interest. For many therapeutic applications, an invasive procedure is needed to inject or implant a drug-eluting device, and these devices cannot be refilled or replaced without another invasive surgery.
As many biological cues are present for specific and short time periods, we have also developed a variety of active scaffolds that can be remotely actuated by ultrasound, electric fields and magnetic fields to deliver factors and cells on demand. In these applications, the microenvironment of the target cells may dramatically impact their ability to respond to the drug, and this is also an active area of investigation.
Overall, our research provides novel therapeutic opportunities for the targeting and treatment of various diseases by controlling spatial arrangement and temporal release of therapeutic agents.
Relevant review articles in this area
- Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071. doi:10.1038/natrevmats.2016.71.
- Kearney CJ, Mooney DJ. Macroscale delivery systems for molecular and cellular payloads. Nat Mater. 2013;12(11):1004-1017. doi:10.1038/nmat3758.
Representative research publications area
- Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551-554. doi:10.1038/9853.
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029-1034. doi:10.1038/nbt1101-1029.
- Chen RR, Silva EA, Yuen WW, et al. Integrated approach to designing growth factor delivery systems. FASEB J. 2007;21(14):3896-3903. doi:10.1096/fj.06-7873com.
- Mooney DJ, Lee KY, Peters MC, Anderson KW. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408(6815):998-1000. doi:10.1038/35050141.
- Brudno Y, Silva EA, Kearney CJ, et al. Refilling drug delivery depots through the blood. Proc Natl Acad Sci. 2014;111(35):12722-12727. doi:10.1073/pnas.1413027111.
- Huebsch N, Kearney CJ, Zhao X, et al. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc Natl Acad Sci. 2014;111(27):9762-9767. doi:10.1073/pnas.1405469111.

